The UK approval process for new treatments
Treatments which are proved through clinical trials to be safe and effective must be approved for use by the UK regulatory body Medicines and Healthcare products Regulatory Agency (MHRA) and also the National Institute for Health and Care Excellence (NICE) before they can be prescribed on the NHS.
The MND Association has worked collaboratively with MND Scotland and My Name’5 Doddie Foundation, with support from Costello Medical, to create the following offering clear, concise information about the UK routes for access to new drugs.
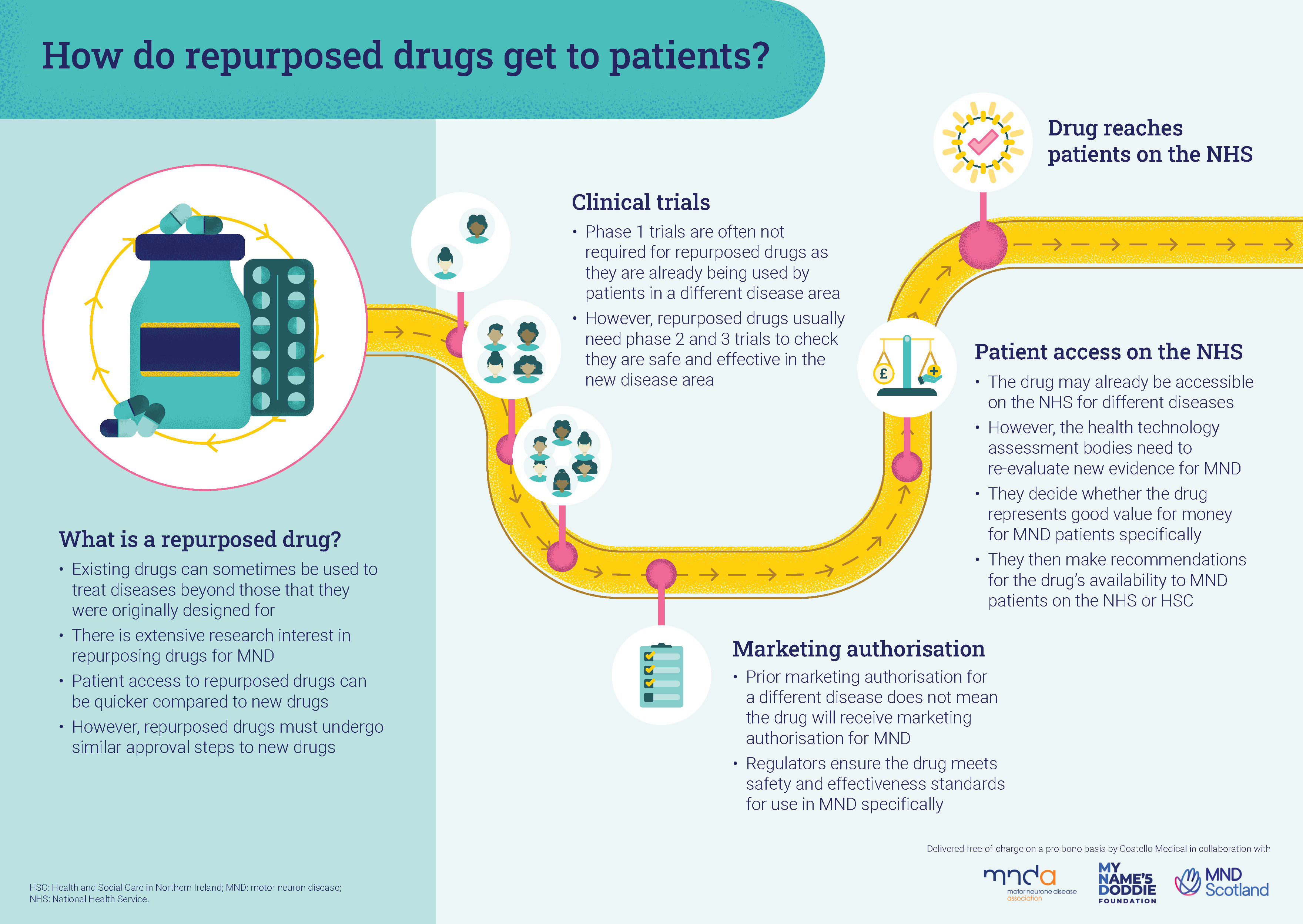
What is the process for repurposed drugs?
The NHS Medicines Repurposing Programme identifies and progresses opportunities to use existing medicines in new ways.
A well-known example is aspirin – it was originally used as a painkiller, but is also now prescribed to reduce the risk of heart attacks and strokes.
The following infographic explains the steps a repurposed drug must go through before being prescribed for a different symptom or illness.
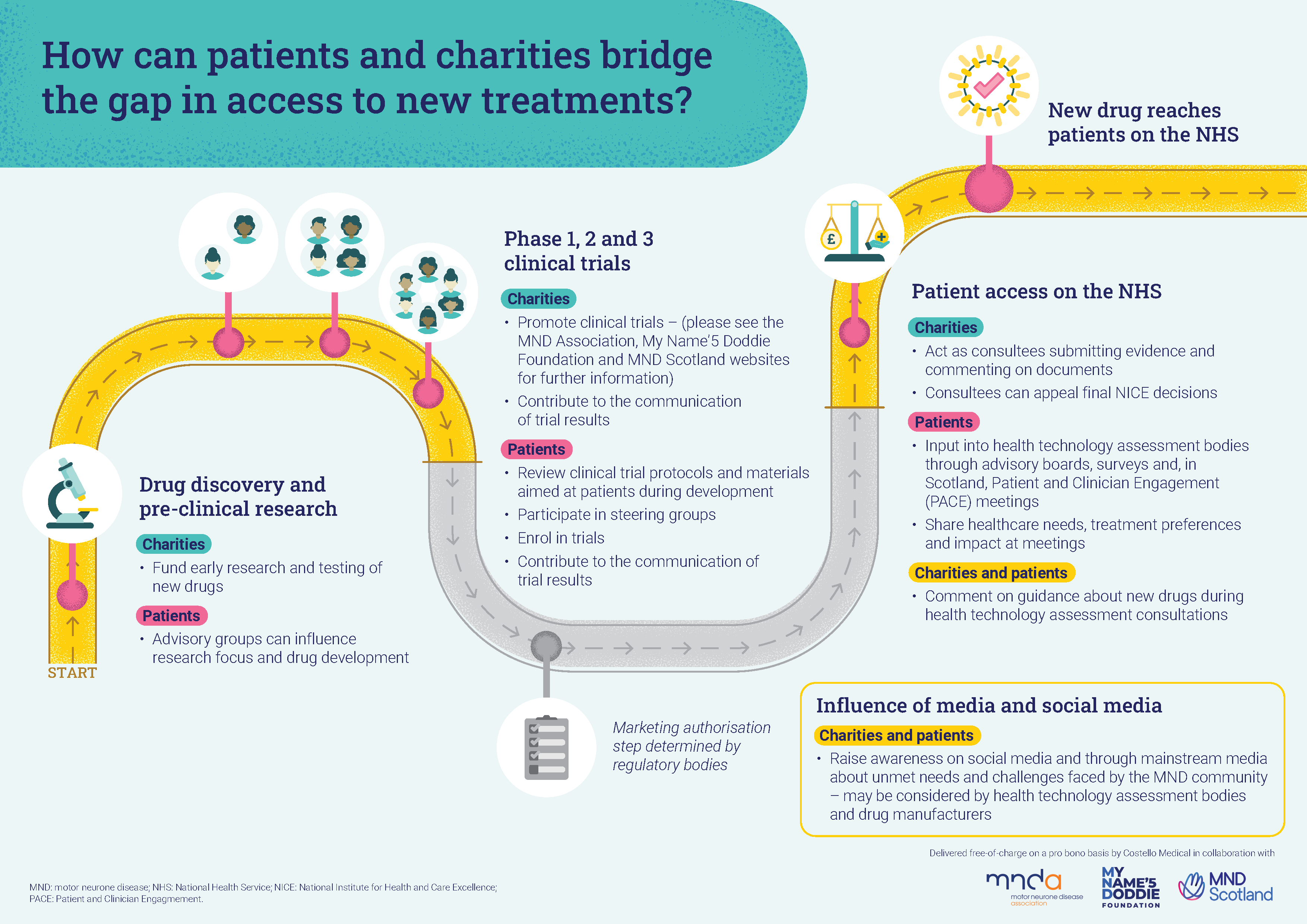
How can people with MND and charities get involved to influence access to treatments?
While the drug development and approval process involves several regulatory bodies and organisations, there are stages where charities and patients can get involved. This includes promoting or taking part in trials, and raising awareness. You can find out more below.
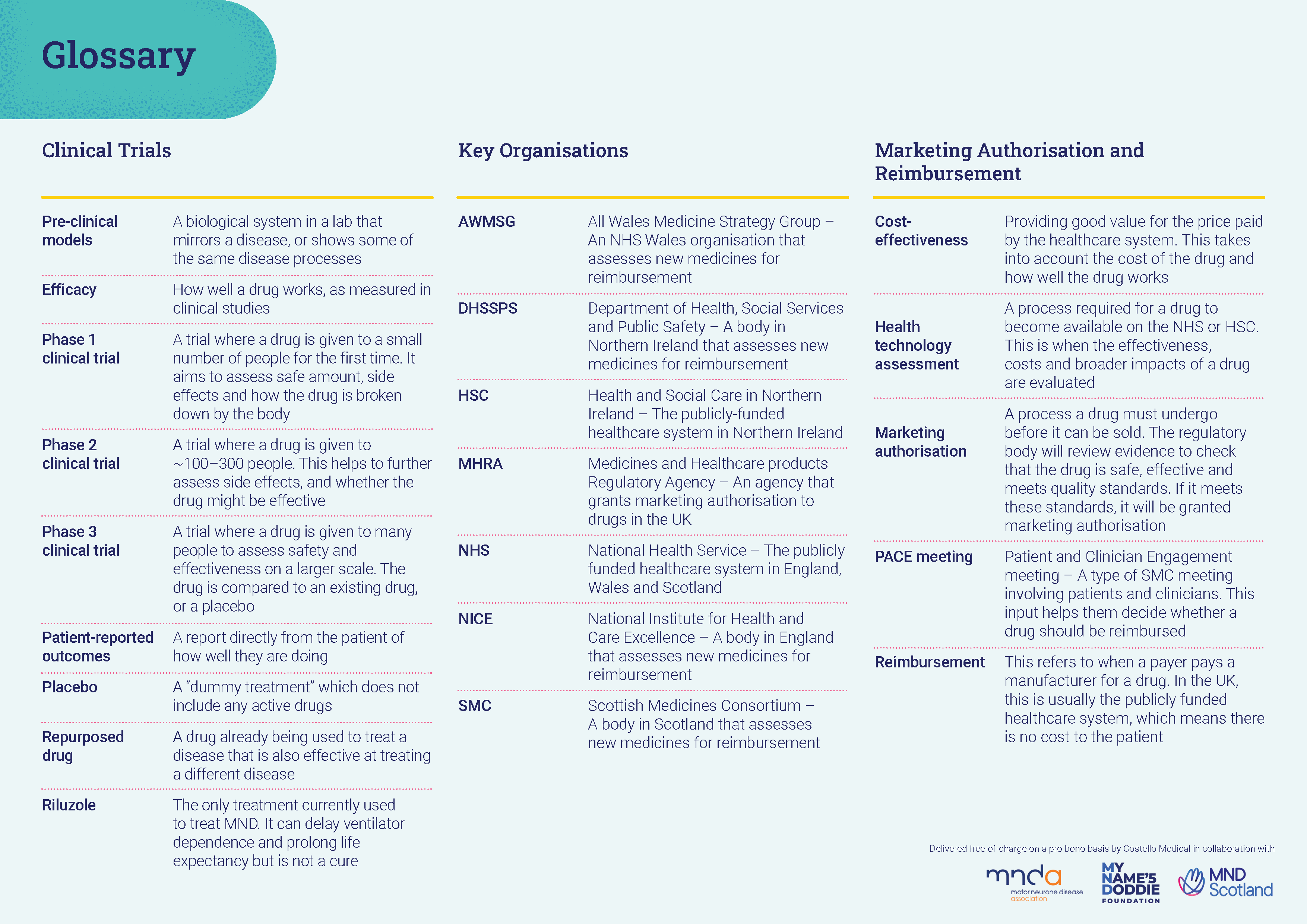
Glossary of terms
Our helpful glossary explains some of the terms mentioned on this page in more detail.